Science
Fibrosis
Fibrosis is estimated to contribute to almost 50% of all deaths in the developed world (Friedman et al., 2013), and despite the existence of drugs that can delay its progression, to date there is no truly curative treatment (Dempsey et al., 2019).
One of the main drivers of fibrosis is the cytokine TGF-β, known as the “Master Regulator of fibrosis”.
TGF-β
TGF-β is a multifunctional cytokine involved in critical processes, including immune regulation and wound healing together with cell proliferation, maturation, and differentiation (Derynck and Budi, 2019). Three TGF-β isoforms have been identified in mammals: TGF-β1, TGF-β2, and TGF-β3.
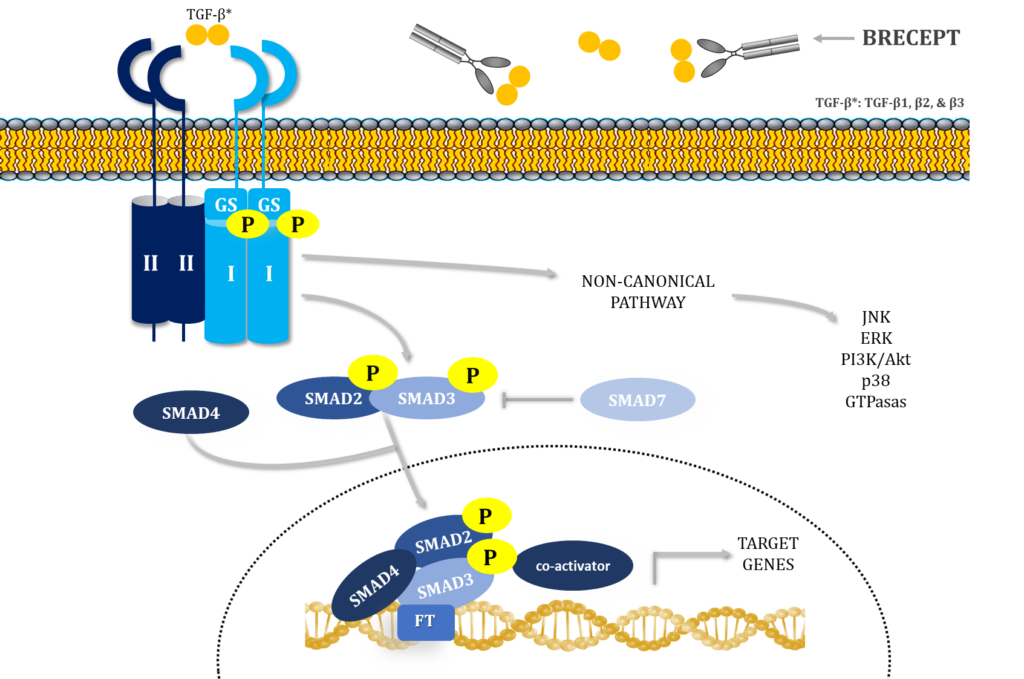
Canonical signaling starts when mature, dimeric TGF-β isoforms bind cell surface receptor complexes comprising “type II” (TβRII) and “type I” (TβRI) receptors (Heldin and Moustakas, 2016). TβRI has a short Gly-Ser-rich juxtamembrane sequence (GS domain) that is phosphorylated by TβRII kinase in response to ligand binding (Wieser et al., 1995; Chaikuad and Bullock, 2016; Heldin and Moustakas, 2016).
Ligand binding to the receptor ectodomains induces conformational changes at the ligand–receptor interface, bringing their cytoplasmic domains closer (Chaikuad and Bullock, 2016; Hinck et al., 2016). This stabilization enables TβRII to phosphorylate TβRI at the serine residues of the GS domain, which then induces conformational changes that activate TβRI kinase (Huse et al., 1999; Chaikuad and Bullock, 2016). TβRI activated by TβRII phosphorylates the two C-terminal serines of Smad2 and 3, inducing their activation. These “receptor-activated Smads” or R-Smads then bind Smad4 and translocate into the nucleus, where they activate or repress target genes (Hata and Chen, 2016).
The highly diverse and context-dependent TGF-β responses are not supported by the canonical signaling model; therefore, it is an oversimplification (Budi et al., 2017; Derynck and Budi, 2019). Also, TGF-β receptors activate non-Smad signaling pathways contributing to the TGF-β response, including pathways such as PI3K-AKT-mTOR and MAPK (Zhang, 2017), adding more complexity to the signaling cascade.
Adding another turn to the TGF-β signaling pathway, here we describe, for the first time, TβRII-SE, a novel human TβRII splice variant that encodes a truncated soluble isoform of the receptor. TβRII-SE isoform binds all three TGF-β ligands, in the picomolar affinity range, without participation of additional receptors.
We fused TβRII-SE with a human IgG1 Fc domain to develop a novel peptibody, called BRECEPT, that neutralizes TGF-β, as a therapeutic for complex chronic diseases with unmet medical needs.
BRECEPT
As a TGF-beta inhibitor, Brecept has demonstrated preclinical antifibrotic activity in Skin, pulmonary, and liver fibrosis; healing effect in skin wound models; and antitumor activity in colorectal and breast cancer models.
Target indications were chosen because fibrosis, aberrant wound healing, and cancer, share many cellular processes such as cell migration, epithelial-mesenchymal transition, and extracellular matrix deposition, as well as a common driver: that is TGF-β.
Scientific resources
– Frontiers in Cell and Developmental Biology
“A Novel Splice Variant of Human TGF-β Type II Receptor Encodes a Soluble Protein and Its Fc-Tagged Version Prevents Liver Fibrosis in vivo”
– SAFIS 2021 Annual Meeting, October 20-22. Virtual platform
“Therapeutic effect of a novel truncated isoform of the human TGF-β type II receptor Fc-tag protein in a liver fibrosis rat model”
La Colla A, Cámara CA, Bertolio MS, Rodríguez TM. Echarte SM, Dewey RA, Chisari AN.
– Meeting of Biosciences Societies (SAIC, SAFIS, SAI) 2020, November 10-13
“A novel soluble isoform of the human TGF-β Type 2 receptor exerts strong antitumor activity in colorectal cancer-derived cell lines”
Romo A, Guo Y, Rodriguez TM, Dewey RA. MEDICINA 2020; 80 (Supl. V): 115.
– Meeting of Biosciences Societies (SAIC, SAFIS, SAI) 2020, November 10-13
“Effect of liver overexpression of Brecept on a rat model of NAFLD”
Cámara CA, La Colla A, Rodríguez TM, Dewey RA, Chisari AN. MEDICINA 2020; 80 (Supl. V): 201.
– Award: “Young Investigators in Physiology”. Granted by the Argentine Society of Physiology (SAFIS)
“Antifibrotic effect on liver of a novel truncated isoform of the human TGF-β type II receptor Fc-tag protein”
La Colla A, Bertolio MS, Campisano S, Rodriguez TM, Camara C, Velasco Zamora J. Dewey Ricardo A, Chisari AN. Juries: María Cecilia Larocca, Gustavo Abraham, Osvaldo Ponzo.
Meeting of the Argentine Society of Physiology (SAFIS), 14–17 November 2018.
– Joint Meeting SAIC, SAI, SAFIS. 14–17 November 2018
“TβRII–SE, a new soluble human TGF-β type II receptor isoform, is sumoylated, interacts with cytoplasmic and nuclear proteins, and is secreted on exosomes”
Marcela Bertolio, Tania Melina Rodriguez, Jorge Velasco Zamora, Marcelo J. Perone, Ricardo A. Dewey. MEDICINA 2018; 78 (Supl. III): 158.
– Award: First Prize for the best Poster of the Regenerative Medicine and Cell Therapy session. Granted by the Argentine Society for Clinical Research (SAIC)
“A novel naturally occurring splice variant of the human TGF-ss type II receptor (TβRII) encodes a truncated soluble molecule”
Marcela Soledad Bertolio, Tania Melina Rodríguez, Alejandra Carrea, Jorge Velasco Zamora, Marcelo Javier Perone, Ricardo Alfredo Dewey. Juries: Dra. Mariana García, Dra. Julieta Maymó, Dr. Leonardo Romorini.
LXI Annual Meeting of the Argentine Society for Clinical Research (SAIC). 15-19 November 2016.
